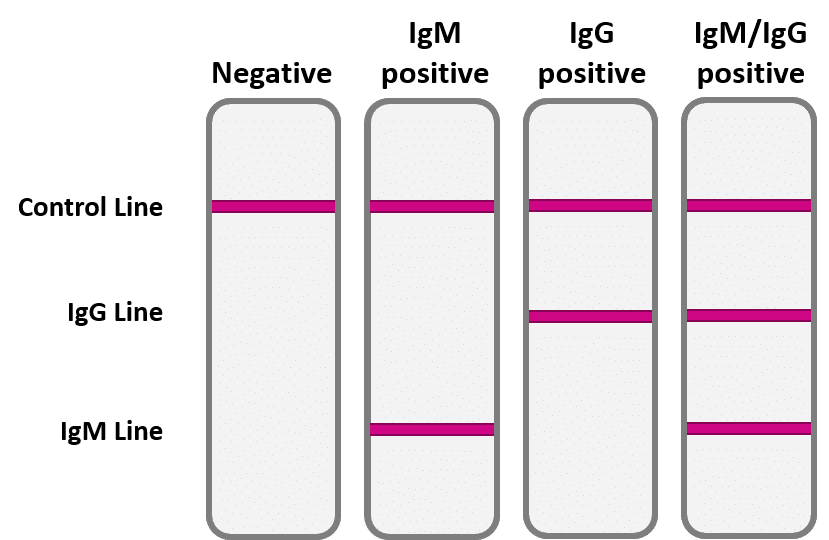
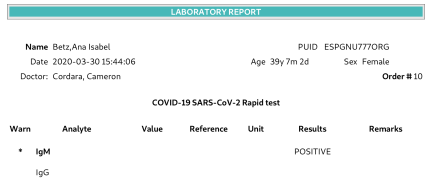
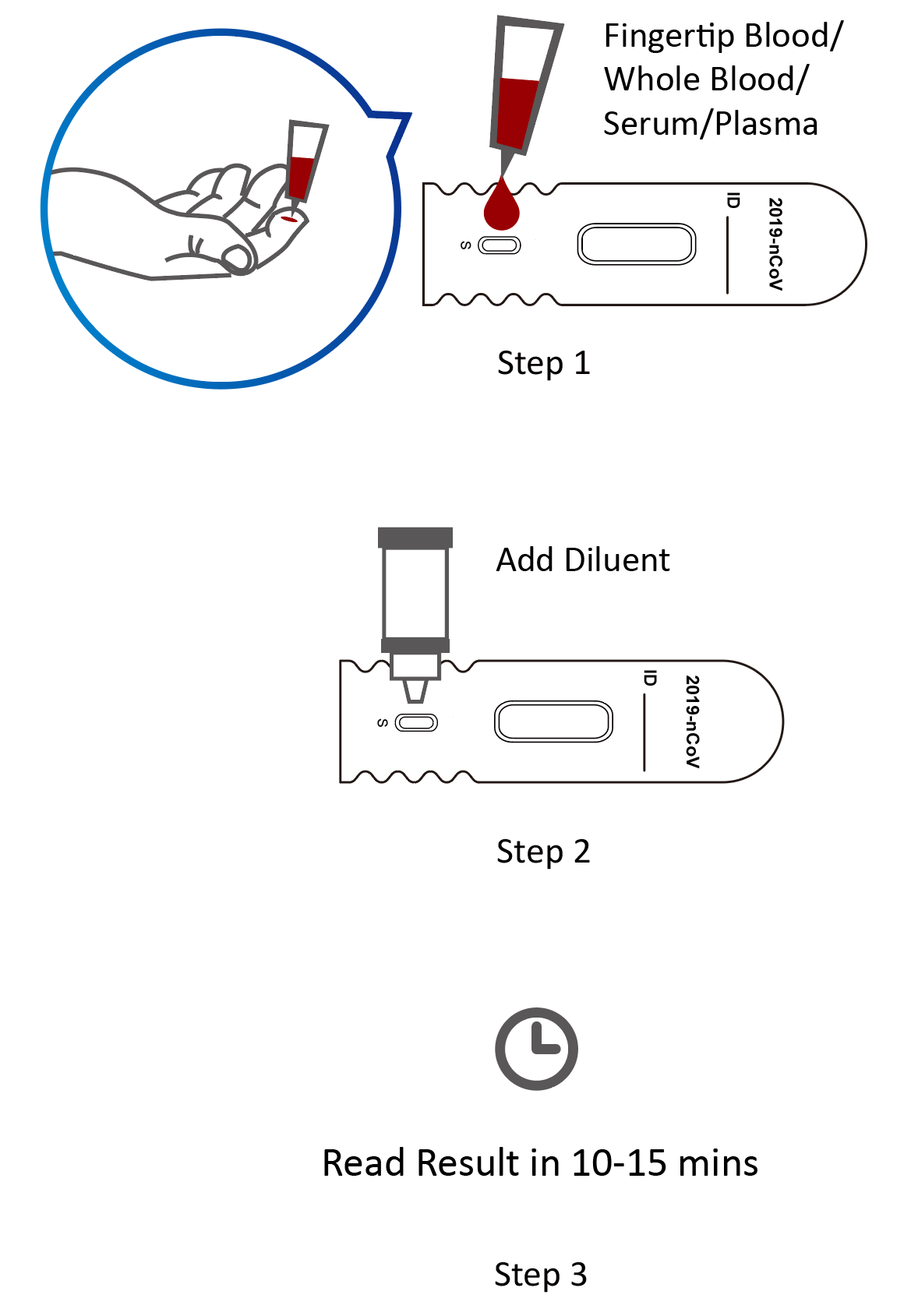
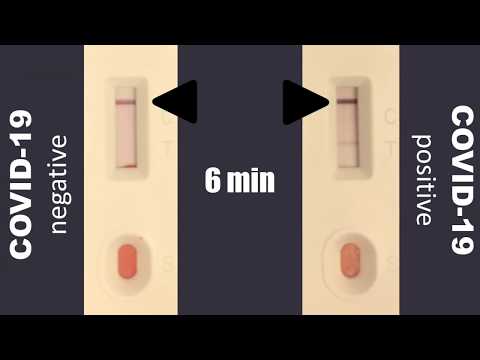
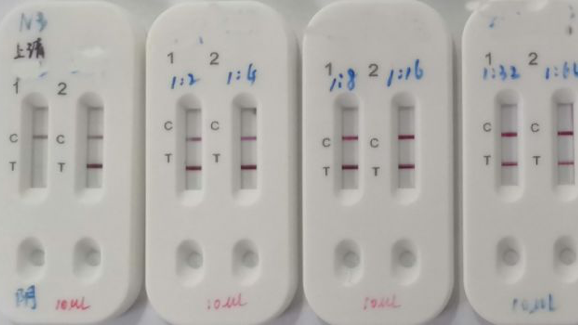
Covid 19 Rapid Test Report Format - Covid-19 Realtime Info
In a facebook post the doh.

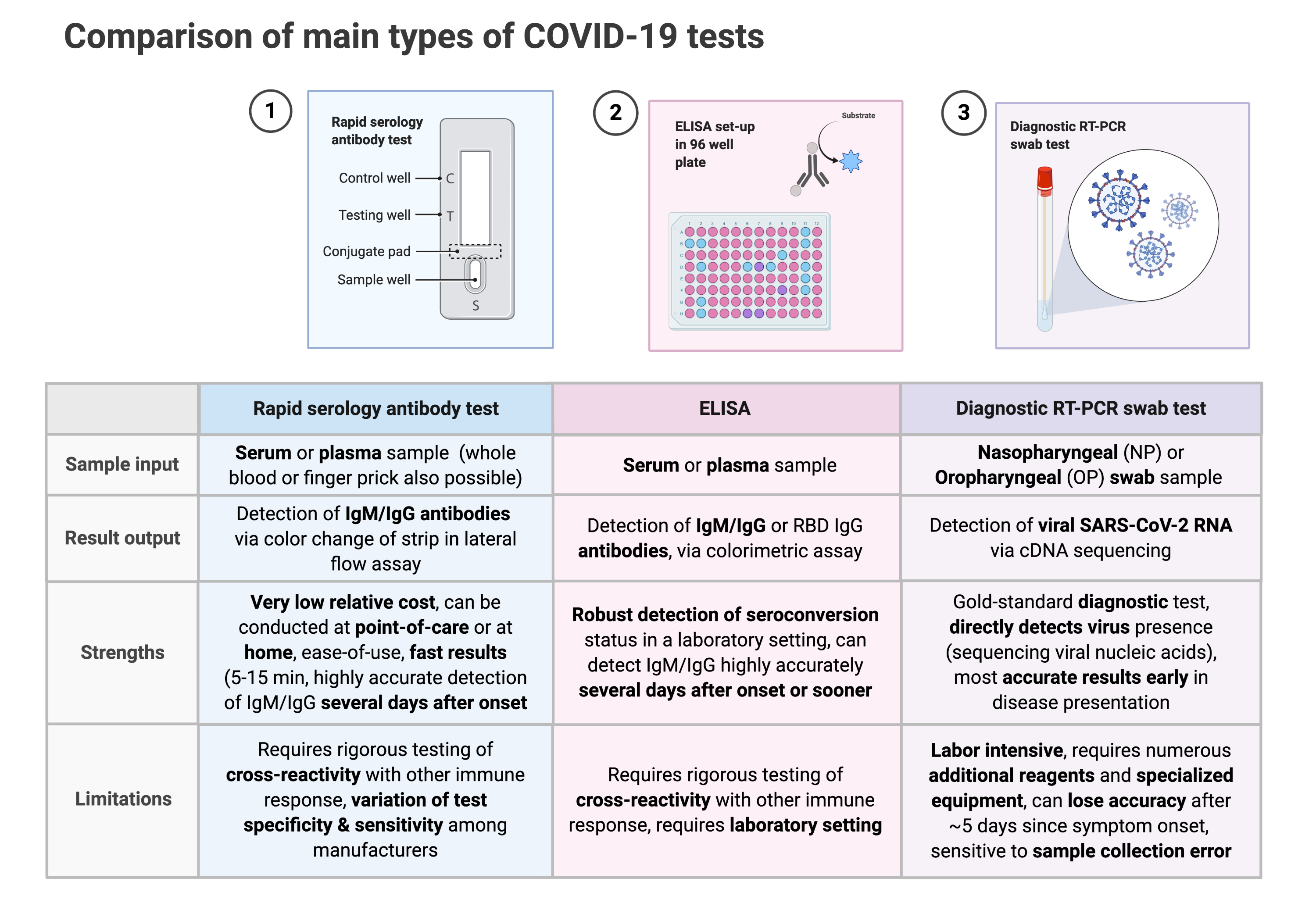
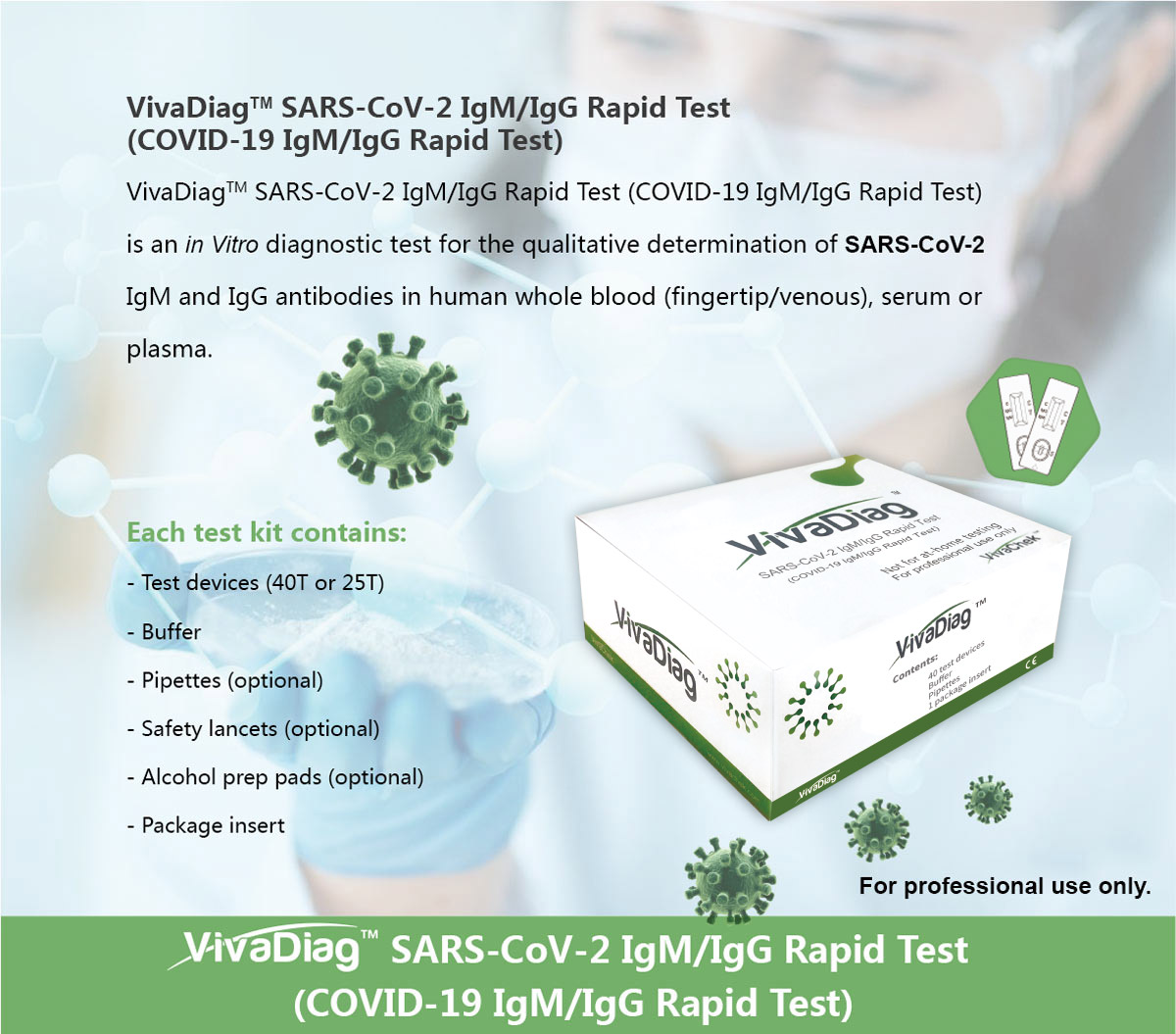
Covid 19 rapid test report format. Abbott laboratories announced wednesday that its new rapid covid 19 antigen test which will cost 5 and provide results in 15 minutes was granted emergency use authorization by the us. Covid 19 assays and test systems used for diagnostic or screening testing including those for antigen testing must have received an eua from fda or be offered under the policies in fdas policy for covid 19 tests external icon. In vitro diagnostic ivd devices are tests performed on samples taken from the human body such as swabs of mucus from inside the nose or back of the. Depending on where you live and whether theres a surge in the number of tests administered can determine how long itll take to get test results.
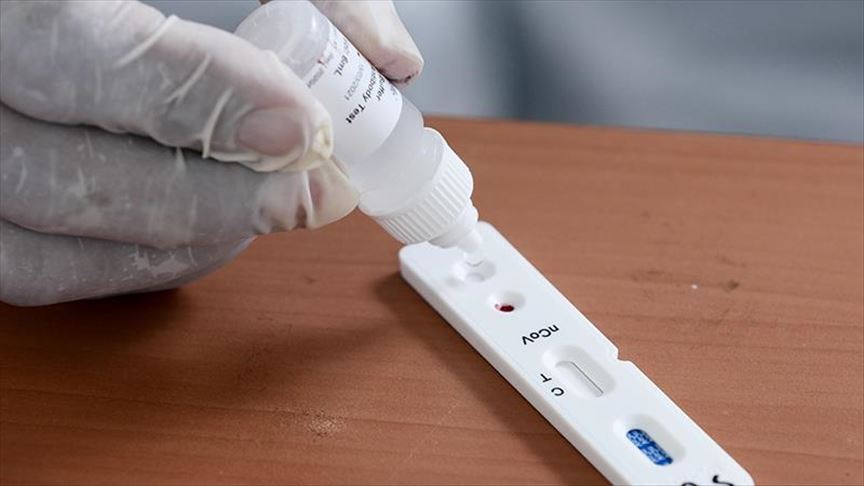
Jakarta kasus positif covid 19 di indonesia terus bertambah. This is the first antigen test that can be read from a testing card similar to a home pregnancy test. Tan shot yen membagikan edukasi mengenai makna rapid test covid 19 melalui media sosialnya. Sebagai tindak pencegahan makin menyebarnya virus corona pemerintah berencana melakukan tes cepat rapid testbeberapa negara sudah melakukan tes cepat untuk mendeteksi virus corona salah satunya australia.
Heres what we know about covid 19 testing right now. The us food and drug administration fda has granted an emergency use authorization eua for a rapid covid 19 test developed by abbott laboratories. Food and drug administration granted emergency use approval wednesday for a credit card sized rapid response covid 19 test made by abbott laboratories which could help to meet the need. In vitro diagnostics euas for covid 19 tests.
Menjawab rasa ingin tahu masyarakat ahli nutrisi dr. Through the antigen. The test costs 5 and results take about 15 minutes. Rapid antigen test reports come under fire in bihar over fake results.
Any rapid antigen test for sars cov 2 authorized for use by fda will be included on fdas list of in vitro. The department of health doh announced that the interim guidelines for using the rapid test kits for coronavirus disease 2019 covid 19 has been released on tuesday.